![]()
When the TIME is right for Immunotherapy
DetermaIO™ is a proprietary gene expression test that assesses the entire Tumor Immune Microenvironment (TIME) to identify the patients most likely to benefit from immunotherapy.
Up to 44%
of all newly diagnosed patients with cancer are eligible for immuno-oncology testing and therapy.1
Unfortunately, with standard of care PD-L1 IHC testing, more than half of PD-L1 positive patients do not respond to immune checkpoint inhibitors, and
1 in 6
patients who will respond are missed.2
better way
DetermaIO
DetermaIO is a novel gene expression assay that measures expression levels of 27-genes to assess various components of the tumor immune microenvironment (TIME). The test has been shown to identify patients that are likely to benefit from immune checkpoint inhibitor (ICI) therapy.
Data showing strong associations between DetermaIO results and benefit from ICI therapy has been published and presented in multiple tumor types including advanced non-small cell lung cancer (NSCLC), triple negative breast cancer (TNBC), urothelial cancer, and colorectal cancer. Additional indications are under investigation.

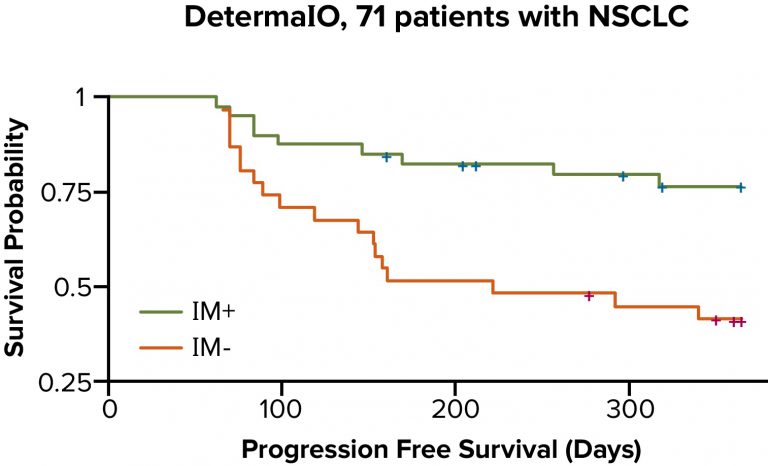
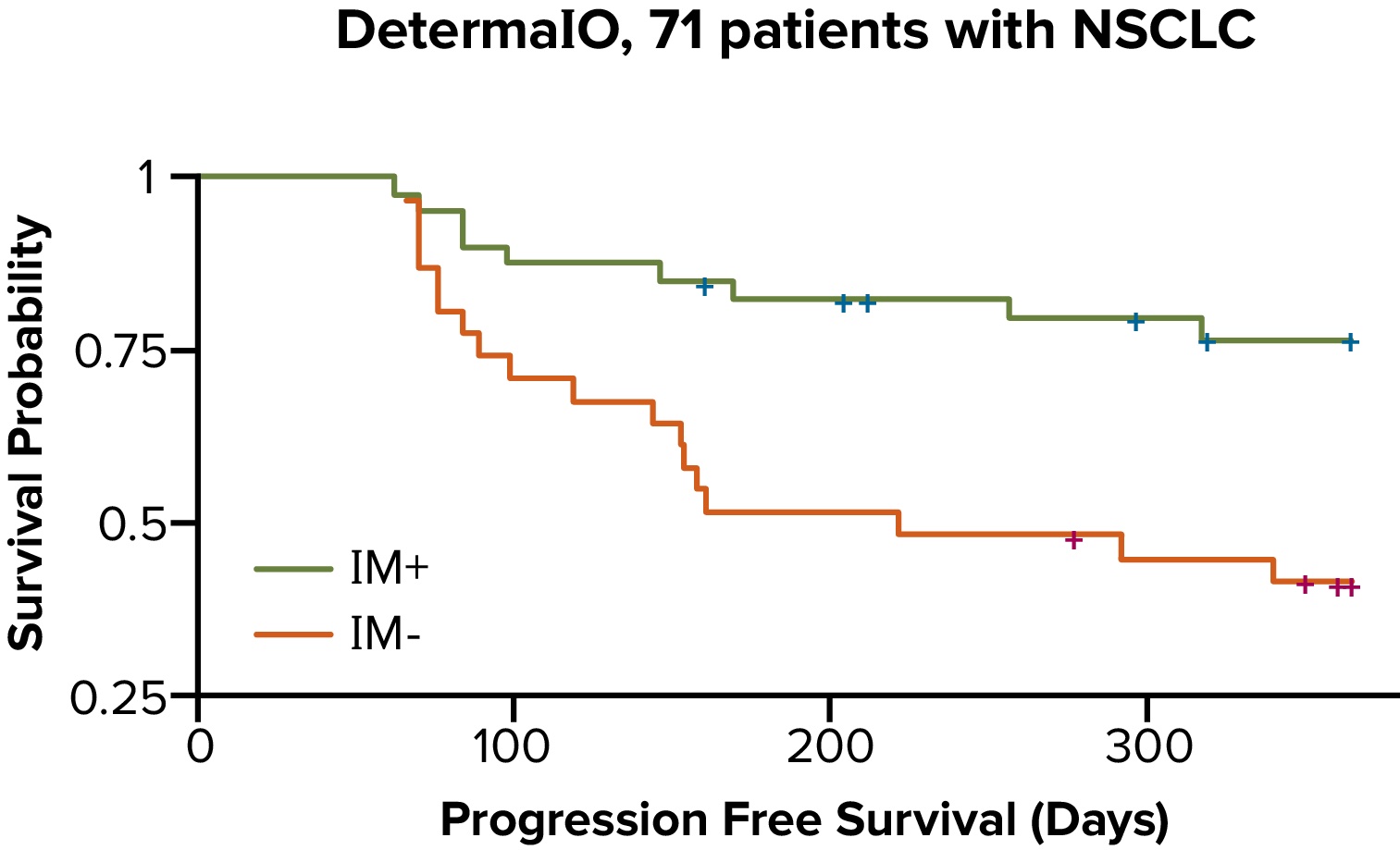
DetermaIO Performance
- In patients with NSCLC treated with an immune checkpoint inhibitor, DetermaIO-identified responders (IM+) had significantly longer progression-free survival than DetermaIO-identified non-responders (IM-).3
- In a study presented at the Society for Immunotherapy of Cancer (SITC) Annual Meeting, data suggested that DetermaIO outperformed PD-L1 in predicting immunotherapy responders as well as non-responders.3
for Research

Effective Results
Standard of care PD-L1 testing incorrectly identifies many patients as potential responders to immune checkpoint inhibitors, and misses certain other patients who may respond.2 Data from a recent study suggested that DetermaIO outperformed PD-L1 in identifying both responders and non-responders.3

Reduce Wasted Spend
Immuno-therapy can cost up to $300,000 per patient.4 Accurately identify the patients that are most likely to respond, reducing expensive drug costs for those that are unlikely to benefit.

Decrease Adverse Events
30% of patients experience a serious adverse event as a result of immune checkpoint inhibitor treatment.2 Avoid introducing potential adverse outcomes (including autoimmune disease) to patients for whom treatment is unlikely to be effective.

Contact Us
References
1. Haslam A, Prasad V, (2019). Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2(5):e192535. 2. Khagi V, Kurzok R, Pravin Patel S, (2017). Next generation predictive biomarkers for immune checkpoint inhibition. Cancer Metastasis Rev 36(1):179-190. 3. Ranganath H, Jain A, Smith J, et al. (2019). One-year progression-free survival in lung cancer patients treated with immune checkpoint inhibitors is significantly associated with a novel immunomodulatory signature but not PD-L1 staining. SITC Annual Meeting, Poster P141, National Harbor, Maryland. 4. Institute for Clinical and Economic Review, (2017). Treatment Options for Advanced Non-Small Cell Lung Cancer: Effectiveness, Value and Value-Based Price Benchmarks. Final Evidence Report and Meeting Summary. Accessed October 2019.
DetermaIO has been developed and its performance characteristics determined by Oncocyte. DetermaIO has not been cleared or approved by the US Food and Drug Administration (FDA). Oncocyte’s laboratory offering DetermaIO is CAP-accredited and CLIA-certified. ©2023 Oncocyte, Inc. All Rights Reserved.