

Blood-based Monitoring
Detect progression early after cancer treatment initiation.
working?
Best decision earlier
DetermaCNI™ testing detects progression early, often after one cycle of treatment, and requires NO Tissue – saving time and significant costs.1
Non-Surgical Candidates
Typically, patients with advanced cancer

- Evaluating early phase therapies
- Targeted therapies
- ICI or novel IO combinations
Resistance and progression
Only a fraction of patients being treated for cancer
respond to treatment.2
Identifying progression early allows physicians to change the course of therapy quickly when time is of the essence. For pharmaceutical companies conducting clinical trials, DetermaCNI can de-risk clinical trials by identifying non-responders early.3
In published studies, DetermaCNI identified progressors early and with high accuracy. Patients with elevated DetermaCNI scores were more than 95% likely to experience clinical progression (>95% PPV).
Sample Patient Reports

DetermaCNI is the only solution that checks all of the boxes

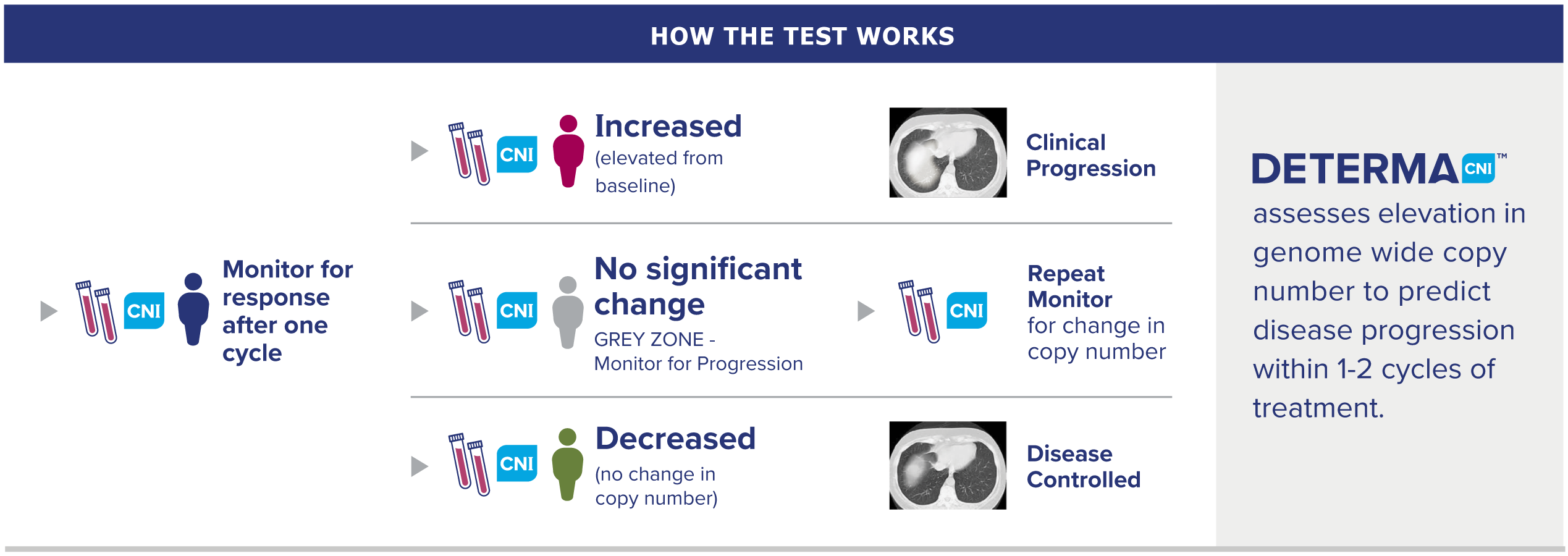
How our test works
References
1. Weiss G, Beck J, Braun D, et al. (2017). Tumor Cell-Free DNA Copy Number Instability Predicts Therapeutic Response to Immunotherapy. Clin Cancer Res 23(17):5074-5081. 2. Weiss G, Blaydorn L, Beck J, et al. (2018). Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs 36(1):96-102. 3. Schirmer M, Beck J, Leu M, et al. (2018). Cell-Free Plasma DNA for Disease Stratification and Prognosis in Head and Neck Cancer. Clin Chem 64(6):959-970. 4. Schütz E, Akbari MR, Beck J, et al. (2015). Chromosomal instability in cell-free DNA is a serum biomarker for prostate cancer. Clin Chem 61(1):239-248.
DetermaCNI has been developed and its performance characteristics determined by Oncocyte. DetermaCNI has not been cleared or approved by the US Food and Drug Administration (FDA). Oncocyte’s laboratory offering DetermaCNI is CAP-accredited and CLIA-certified. ©2023 Oncocyte, Inc. All Rights Reserved.


