
Confidently monitor your kidney transplant patients with VitaGraft™ Kidney.
Fast turnaround time. Improved outcomes.
VitaGraft KidneyTM lets you know sooner to act faster
Practical. Fast. Evidence-based.
Rule out suspected injury
86% of unnecessary biopsies triggered by elevated plasma creatinine in stable patients may be avoided using VitaGraft Kidney dd-cfDNA technology.1
Taper immunosuppression
Better personalize the minimum effective dose for each patient.1
Detect injury early
Use VitaGraft Kidney with improved turnaround times in for-cause
clinical scenarios.1
Pioneering patient care together. Clear, concise results.
VitaGraft Kidney
is a non-invasive, blood-based transplant monitoring test that quantifies the concentration of donor-derived cell-free DNA (dd-cfDNA) following kidney transplantation.

- Optimized turnaround time
- Negative predictive value (NPV) of 91%*1
- Highly precise droplet-digital polymerase chain reaction (ddPCR) technology
- A personalized assay for each patient
- Absolute and fractional quantification reported
*Calculate at a 25% prevalence using biopsy-proven samples.
Process overview
Step 1
Post-transplant
patient blood
sample drawn*

Step 2
Send samples to Oncocyte’s CAP/CLIA certified laboratory

Step 3
dd-cfDNA measured using dd-PCR

Step 4
Risk for active
rejection reported

*The initial test requires a one-time urine sample in addition to blood to set the assay for the patient. Thereafter, we require blood only for all subsequent tests.
Manage the risk of transplant rejection
VitaGraft Kidney technology
Donor-derived cfDNA is used as a biomarker to assess organ health and rejection risk status. Through ddPCR, VitaGraft Kidney determines a set of single-nucleotide polymorphisms (SNPs) specific to each patient.2
A subset of SNPs are selected from “scientifically-validated” commonly-shared SNPs across the global population. This allows for differentiation between the donor and recipient cfDNA. Using the selected personalized assay, dd-cfDNA is measured using absolute and relative quantification.2
With VitaGraft Kidney’s strong performance of 92% diagnostic specificity, 91% NPV,* and fast
turnaround time, injury can be assessed with confidence1.
*Calculated at a 25% prevalence using biopsy-proven samples.
Organ health and dd-cfDNA

See our
Clinical evidence
- Validation Data
- Guide Immunosuppression
- Absolute Quantification Performance
Receiver Operating Characteristic (ROC) curve comparison of dd-cfDNA (cp/mL) and dd-cfDNA (%) shows superior performance of absolute amount1
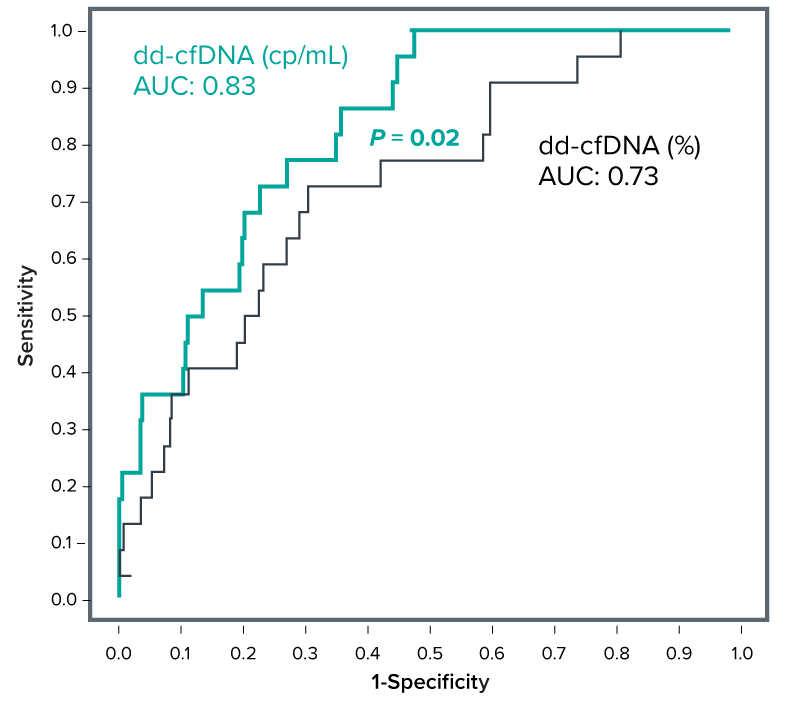
- Validated in 345 kidney transplant patients with over 2,570
samples for up to 5 years following transplantation1,3 - For long-term surveillance, measurement of absolute dd-cfDNA concentrations appears to be superior to percentages to minimize false positive results1,3
- Area Under the ROC (AUCROC) curve for absolute quantity of dd-cfDNA (cp/mL) showed an increase of 14% compared to relative quantification of dd-cfDNA (%) in discriminating stable phase (n=395) from biopsy-proven rejection (n=22)1
- High NPV of 91%* to rule out injury1
*Calculated at a 25% prevalence using biopsy-proven samples.
n = number of samples
Association between increased dd-cfDNA values and low tacrolimus concentrations following kidney transplantation1
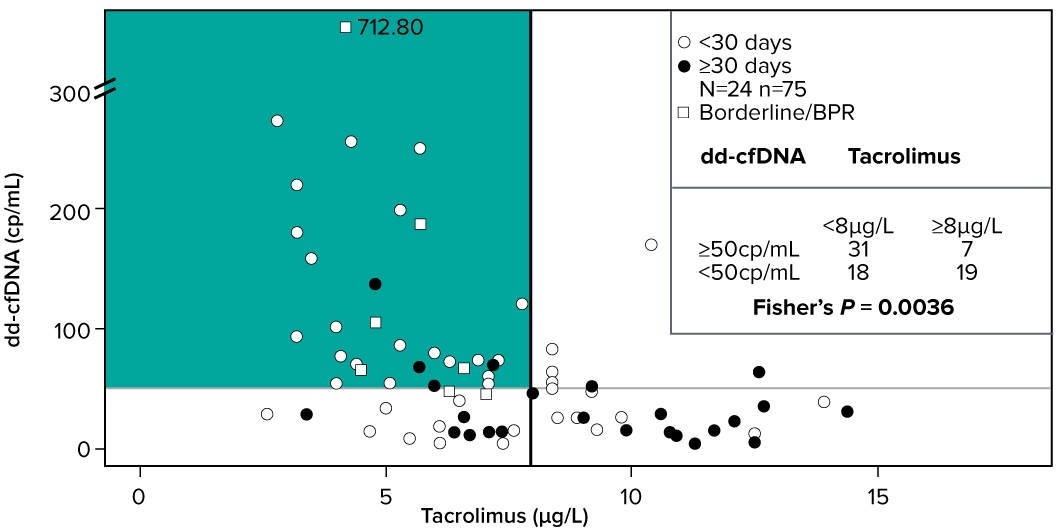
- dd-cfDNA (cp/ml) may aid clinical care in finding the minimum effective immunosuppressive dose for kidney transplant patients1
- There were a significantly higher proportion of samples with elevated dd-cfDNA (cp/mL) and lower tacrolimus levels (<8µg/L) compared to the samples with higher tacrolimus concentrations1
Time-related stability of dd-cfDNA (cp/mL) compared to other markers for up to five years following kidney transplantation3
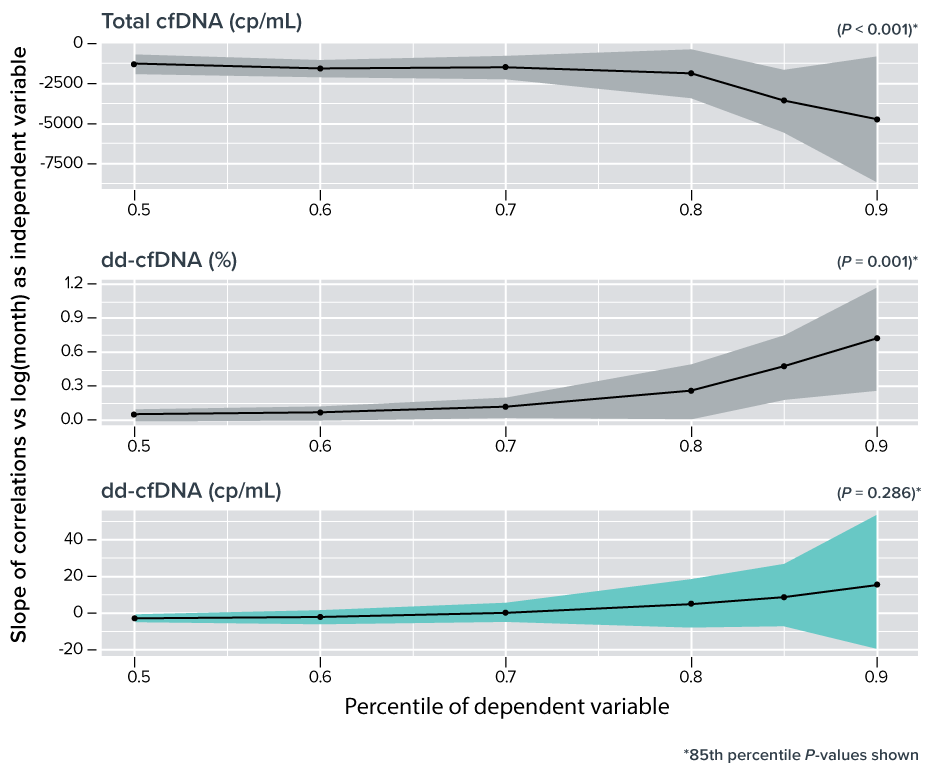
- Absolute dd-cfDNA (cp/mL) remained stable during the 12-60 month study period, along with median plasma creatinine and estimated Glomerular Filtration Rate (eGFR), confirming that absolute quantification accurately indentified the 303 stable kidney transplant patients3
- Total cfDNA median values steadily decreased over time resulting in statistically significant increased dd-cfDNA (%) that was independent of graft health3
- This decrease can be explained by a pharmacological effect of reducing the dosage of calcineurin inhibitors (CNIs) (e.g., tacrolimus)3
VitaGraft resource library
Kidney Clinical Summary
Oellerich M, et al. (2019)
Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: Results from a prospective observational study. Am J Transplant 19(11):3087.
Kidney Clinical Summary
Schütz E, et al. (2020)
Time-dependent apparent increase in dd-cfDNA percentage in clinically stable patients between one and five years following kidney transplantation. Clin Chem 66(10):1290.
Kidney Information Sheet
Intro to VitaGraft Kidney
For nephrologists, surgeons, and clinical transplant professionals.
FAQs
Test We identify informative SNPs selected from a set of predefined SNPs. The informative SNP set for each patient is defined on the first-contact sample (selection). For all subsequent tests (monitoring), we measure the concentration of dd-cfDNA and provide a rejection risk report.
Order VitaGraft Kidney for your patients following kidney transplantation. Reach out to Oncocyte’s Customer Service for ordering details.

We’re here to help
Oncocyte Customer Service can answer any questions you have. Please contact us at:
Phone: 844-621-8880
[email protected]
We do not want cost to be a barrier for testing, patients can call (844-621-8880) or fax (844-584-3467) to see if they qualify for our financial assistance program.
References
1. Oellerich M, Shipkova M, Asendorf T, et al. (2019) Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: Results from a prospective observational study. Am J Transplant 19(11):3087. 2. Beck J, Bierau S, Balzer S, et al. (2013) Digital droplet PCR for rapid quantification of donor DNA in the circulation of transplant recipients as a potential universal biomarker of graft injury. Clin Chem 59(12):1732. 3. Schutz E, Asendorf T, Beck J, et al. (2020) Time-dependent apparent increase in dd-cfDNA percentage in clinically stable patients between one and five years following kidney transplantation. Clin Chem 66(10):1290.
©2023 Oncocyte, Inc. All Rights Reserved. The VitaGraft Kidney Test has been developed and its performance characteristics determined by Oncocyte. The VitaGraft Kidney Test has not been cleared or approved by the US Food and Drug Administration (FDA). Oncocyte’s laboratory offering the VitaGraft Kidney Test is CAP-accredited and CLIA-certified.
