
The immune system is smart.
VitaGraftTM Kidney is SMARTER.
S
Specific
Confidently rule out injury with a 94% NPV*1,2
M
Measurable
Quantify dd-cfDNA using both fractional
abundance (%) & absolute quantification (cp/mL)
A
Actionable
dd-cfDNA has a short half-life (20min - 2hrs), allowing almost immediate detection of injury & recovery3
R
Reimbursed
VitaGraft Kidney is covered by Medicare
T
Timely
Receive results typically within 48 hours** to optimize patient management decisions
E
Easy to use
Seamlessly integrate into current workflows
R
Reliable
The only reimbursed test on the market that uses dd-PCR to quantify dd-cfDNA***
*Calculated at 25% prevalence using biopsy-proven samples from combined data from references 1,2.
** Upon sample receipt.
*** dd-PCR is used to calibrate NGS, see references 3-5
VitaGraft Kidney lets you know sooner to act faster
Practical. Fast. Evidence-based.
Pioneering patient care together
VitaGraft Kidney
is a blood-based transplant monitoring test that quantifies the concentration of donor-derived cell-free DNA (dd-cfDNA) following kidney transplantation.

- Optimized turnaround time
- Negative predictive value (NPV) of 94%*1,2
- Highly precise droplet-digital polymerase chainreaction (dd-PCR) technology
- A personalized assay for each patient
- Absolute and fractional quantification reported
*Calculated at a 25% prevalence using biopsy-proven samples.
Process overview
Step 1
Post-transplant patient blood sample drawn at transplant facility or local laboratory*
Step 2
Send samples to Oncocyte’s CAP/CLIA certified laboratory

Step 3
dd-cfDNA measured using dd-PCR
Step 4
Risk for active rejection reported

*The initial test requires a one-time urine sample in addition to blood to set the assay for the patient. Thereafter, we require blood only for all subsequent tests.
Manage the risk of transplant rejection
VitaGraft Kidney technology
Donor-derived cfDNA is used as a biomarker to assess organ health and rejection risk status. Through dd-PCR, VitaGraft Kidney determines a set of single-nucleotide polymorphisms (SNPs) specific to each patient.6
A subset of SNPs are selected from commonly-shared SNPs across the global population. This allows for dierentiation between the donor and recipient cfDNA. Using the selected personalized assay, dd-cfDNA is measured using absolute and relative
quantification.6
With VitaGraft Kidney’s strong performance of up to 98% diagnostic specificity, up to 94% NPV,* and fast turnaround time, injury can be assessed with confidence.1,2
*Calculated at a 25% prevalence using biopsy-proven samples.
Organ health and dd-cfDNA
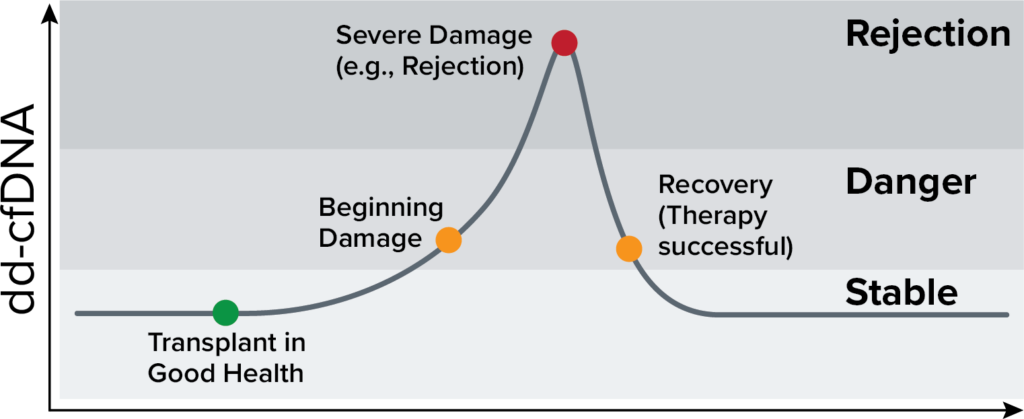
Time after transplantation
An increase in dd-cfDNA may indicate rejection
See our
Clinical evidence
Validated in 459 kidney transplant
patients with over 3,000 samples
for up to five years post-transplant.1,2,7,8
Receiver Operating Characteristic (ROC) curve comparing biopsy-proven rejection (BPAR) to biopsy-proven non-rejection in kidney transplant patients using dd-cfDNA (cp/mL) and dd-cfDNA (%)1,2
Validated in two independent studies
VitaGraft Kidney has been shown to significantly discriminate between rejection and non-rejection using biopsy-proven samples.1,2
Performance metrics for VitaGraft Kidney
*Decision limits for % and cp/mL.
**Calculated using biopsy-proven samples from combined data from references 1,2.
VitaGraft Kidney results quantify dd-cfDNA using both % and cp/mL to give the most information possible
VitaGraft resource library
For providers
Kidney Clinical Summary
Mayer KA, et al. (2024)
A Randomized Phase 2 Trial of Felzartamab in Antibody-Mediated Rejection. NEJM DOI: 10.1056/NEJMoa2400763.
Kidney Clinical Summary
Akifova A, et al. (2023)
Donor-Derived Cell-Free DNA in Biopsy-Proven Antibody-Mediated Rejection Versus Recurrent IgA Nephropathy After Kidney Transplantation. Kidney International Reports. Vol. 8, Issue 10, P2141-2145.
Kidney Clinical Summary
Oellerich M, et al. (2019)
Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: Results from a prospective observational study. Am J Transplant 19(11):3087.
Kidney Clinical Summary
Schütz E, et al. (2020)
Time-dependent apparent increase in dd-cfDNA percentage in clinically stable patients between one and five years following kidney transplantation. Clin Chem 66(10):1290.
Kidney Information Sheet
Intro to VitaGraft Kidney
For nephrologists, surgeons, and clinical transplant professionals.
For patients
VitaGraft Kidney Patient Guide
For patients and caregivers seeking more information on VitaGraft Kidney, how it may help, and where it fits into post-kidney transplant care.
VitaGraft Kidney Patient Billing Brochure
For information on insurance coverage for VitaGraft Kidney.
VitaGraft Kidney Patient Billing Brochure - Spanish (Guía de Facturación Para Pacientes)
Para obtener información sobre la cobertura del seguro para VitaGraft Kidney.
FAQs
- Oncocyte Main Phone: 949-409-7600
- Customer Experience Information:
- Phone: 844-621-8880
- Fax: 844-584-3467
- Email: [email protected]
- CAP/CLIA-Certified Laboratory in Nashville, TN:
- Monday – Friday: 6:30am CST – 12am CST
- Saturday: 8am CST – 6pm CST
- Customer Experience Team:
- 8am EST – 5pm EST
- VitaGraft is a blood-based transplant monitoring test that quantifies the concentration of donor-derived cell-free DNA (dd-cfDNA) following liver and kidney transplantation.
- Increased amounts of dd-cfDNA following transplantation may indicate organ damage and possible rejection.
- VitaGraft is not indicated in patients who are: pregnant, less than two weeks post-transplant for
subsequent testing, recipients of an allograft from an identical twin, bone marrow transplant recipients,
recipients of an allogeneic stem cell transplant, recipients of a non-liver or non-kidney organ transplant,
or recipients of multiple organ transplants.
- VitaGraft Liver and VitaGraft Kidney have different diagnostic thresholds (i.e., the amount of dd-cfDNA that may indicate organ damage differs between the two organs).
- Kidney diagnostic thresholds: 0.5% and 50cp/mL
- Liver diagnostic thresholds: 10% and 1000cp/mL
- Although both tests quantify dd-cfDNA using blood, VitaGraft Kidney requires a urine sample for the initial test to set the assay for the patient. Thereafter, we require blood only for all subsequent tests.
- Blood samples must be processed within 7 days of collection.
- Less than 48 hours within specified hours of operation.
- Through the online portal or by filling out a paper Test Requisition Form (TRF). Please contact your Local Representative or Oncocyte Customer Experience (844-621-8880) for any questions.
- We offer zero interest payment plans for balances over $99. The payment plans are for 3 to 6 months.
- An EOB is a statement from your health insurance plan describing what costs it will cover for medical care or products you’ve received. It is not a bill.
- Patients can make a payment by check or credit card (accepted types: VISA, Mastercard, and Discover). We accept credit card payments over the phone. Checks can be sent to the lock box address: Oncocyte Corporation, PO Box 120293, Dallas, TX 75312-0293. We send statements with the mailing address and phone numbers to the patient every 30 days for 3 cycles.
Test We identify informative SNPs selected from a set of predefined SNPs. The informative SNP set for each patient is defined on the initial contact sample. For all subsequent tests, we measure the concentration of dd-cfDNA and provide a rejection risk report.
Order VitaGraft Kidney for your patients following kidney transplantation. Reach out to Oncocyte's Customer Service for ordering details.
We’re here to help
Oncocyte Customer Service can answer any questions you have. Please contact us at:
phone: 844-621-8880
[email protected]
We do not want cost to be a barrier for testing, patients can call (844-621-8880) or fax (844-584-3467) to see if they qualify for our financial assistance program.
REFERENCES: 1. Oellerich M, Shipkova M, Asendorf T, et al. (2019) Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: Results from a prospective
observational study. Am J Transplant 19(11):3087. 2. Akifova A, Budde K, Choi M, et al. (2023). Donor-Derived Cell-Free DNA in Biopsy-Proven Antibody-Mediated Rejection Versus Recurrent IgA Nephropathy After Kidney Transplantation. Kidney International Reports doi:10.1016/j.ekir.2023.07.011. 3. Oellerich M, Sherwood K, Keown P, et al. (2021). Liquid biopsies: donor-derived cell-free DNA for the detection of kidney allograft injury. Nat Rev Nephrol 17(9):591. 4. Altug Y, Liang N, Ram R, et al. (2019). Analytical Validation of a Single-nucleotide Polymorphism-based Donor-derived Cell-free DNA Assay for Detecting Rejection in Kidney Transplant Patients. Transplantation 103(12):2657. 5. Grskovic M, Hiller DJ, Eubank LA, et al. (2016) Validation of a Clinical-Grade Assay to Measure Donor-Derived Cell-Free DNA in Solid Organ Transplant Recipients. J Mol Diagn 18(6):890. 6. Beck J, Bierau S, Balzer S, et al. (2013) Digital droplet PCR for rapid quantification of donor DNA in the circulation of transplant recipients as a potential universal biomarker of graft injury. Clin Chem 59(12):1732. 7. Schutz E, Asendorf T, Beck J, et al. (2020) Time-dependent apparent increase in dd-cfDNA percentage in clinically stable patients between one and five years following kidney transplantation. Clin Chem 66(10):1290. 8. Osmanodja B, Akifova A, Budde K, et al. (2021). Absolute or Relative Quantification of Donor-derived Cell-free DNA in Kidney Transplant Recipients: Case Series. Transplant Direct 7(11):e778.
© 2024 Oncocyte Corporation. All Rights Reserved. The VitaGraft Kidney Test has been developed and its performance characteristics determined by Oncocyte. The VitaGraft Kidney Test has not been cleared or approved by
the US Food and Drug Administration (FDA). Oncocyte's laboratory oering the VitaGraft Kidney Test is CAP-accredited and CLIA-certified.
